assay of barium sulphate by gravimetric method|barium sulfate concentration : manufacturing A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the hot solution. The precipitate is filtered through a paper filter which is then ignited and . web1 de jan. de 2024 · Bomba Patch 2024, atualizada em janeiro! Faça o download agora mesmo da ISO e desfrute das melhores atualizações, novidades exclusivas e aprimoramentos no jo.
{plog:ftitle_list}
20 de agosto de 2020. Crédito da foto: Divulgação. Único brasileiro campeão Overall do campeonato amador mais difícil do mundo, o Arnold Classic Ohio em (Columbus, Ohio EUA), Fabio Giga virou pro este ano .
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the hot solution. The precipitate is filtered through a paper filter which is then ignited and .
In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will . This document describes a procedure for determining the amount of sulfate in an unknown sample using gravimetric analysis with barium sulfate precipitation. Key steps include: 1) Adding barium chloride to the sample to .The document describes a procedure to estimate the amount of barium in a sample of barium sulfate using gravimetric analysis. An unknown sulfate salt is dissolved and reacted with barium chloride, precipitating out barium sulfate. . In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will be determined by gravimetric analysis. First, a pre-weighed sample of the unknown .
Quantification of total sulfur can be done based on gravimetric analysis of precipitated barium sulfate in solution. METHODS FOR SULFATE QUANTIFICATION IN SOILS A wide range of test methods are currently used .By EVELYN M. COCHRAN, ERVIN B. INSKIP, PERRY KING, and HOWARD W. ZIEGLER. A method suitable for compendia1 assay of barium sulfate USP has been developed. Barium is .Gravimetric Determination of Soluble Sulfate. The quantitative determination of sulfate ions in inorganic compounds can be accomplished by using the selective precipitation of the sulfate .
gravimetric sulfate testing
This video (Gravimetric titration part 2 | Assay of barium sulphate (BaSO4)) contains basic principle, reaction, procedure as well as calculations involved i.GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown .In this test method the sulfate ion is precipitated with barium chloride, in an acidic medium, to barium sulfate crystals of uniform size. The barium sulfate present in suspension is determined by a measurement of its turbidity and comparison with a known . “Gravimetric Method”; and the ASTM Designation: D 516, “Sulfate Ion in Water”.Quantification of total sulfur can be done based on gravimetric analysis of precipitated barium sulfate in solution. METHODS FOR SULFATE QUANTIFICATION IN SOILS A wide range of test methods are currently .
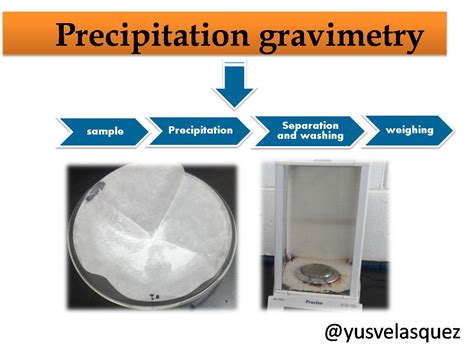
established analytical methods we consider this term. Precipitation Gravimetry Gravimetric analysis is a standard classical method for determining the amount of a given component present in a host of solid and solution sample types. The method used here involves precipitating the component of interest from the unknown by means of an added reagent.
Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. . Put your understanding of this concept to test by answering a few MCQs .The sample containing sulfate is then reacted with an alcohol solution of barium chloride and methylthymol blue (MTB) at a pH of 2.5-3.0 to form barium sulfate. The combined solution is raised to a pH of 12.5-13.0 so that excess barium reacts with MTB. The uncomplexed MTB color is gray; if it is all chelated with barium, the color is blue.Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of .Thermo gravimetric analysis method of analysis includes quantitative change in mass of analyte with reference to temperature. This technique is purely instrumental. . 4.2 Determination of sulphate as barium sulphate: . Test the supernatant liquid for complete precipitation by adding a few drops of barium chloride solution. Add barium .
Sodium sulphate for industrial use — Determination of sulphates content — Calculation method and barium sulphate gravimetric method. Skip to main content. Practical guidance on the SDGs . extraction of a test portion with dilute acid, separation of the insoluble matter, precipitation of the sulphate in the filtrate with barium chlorid and .A method suitable for compendial assay of barium sulfate USP has been developed. Barium is separated as the carbonate and precipitated as the chromate. Calcium, strontium, and silica do not interfere. The calcium and strontium content may be determined by atomic absorption or X-ray fluorescence and silica by a colorimetric procedure.for the determination of sulfate. Which indication method is the most suitable depends above all on the sample matrix. Method 1: Precipitation as barium sulfate and back-titration of the Ba2+ excess with EGTA. The ion-selective calcium electrode is used as indicator electrode. Method 2: As in method 1, but with the electrode combination .
This standard prescribes the following methods for determination of various elements in manganese ores: a) Chemical analysis by gravimetric and volumetric method, and b) Atomic absorption method. The gravimetric and volumetric methods are suitable for determination of silica, barium oxide, manganese, iron, phosphorus, sulphur, alumina and other .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed . B . It is used to assay barium sulphate. C. It is used to assay of aluminum. D. Relative precision 3 to 4%. 3. Which sentence is true about a gravimetric analysis ? A.Relative precision 3 to 4%. B. It is expansive method. C. It is not used to to barium sulphate assay. D. It is used to assay of Al. 3. How many Types of gravimetric analysis ? A .
2. Gravimetric Method 2.1 Scope and Application -This method is applicable for all the waters having sulphate concentrations above 10 mg/l; however, it is a time consuming method. 2.2 Principle and Theory - Sulphate is precipitated in hydrochloric acid medium as barium sulphateISO 2480:1972 Sodium chloride for industrial use — Determination of sulphate content — Barium sulphate gravimetric method. Published (Edition 1, 1972) This publication was last reviewed and confirmed in 2023. . The principle consists in dissolution of a test portion and separation of the insoluble residue. Precipitation of the sulphate .A gravimetric method in which the . mass of a particulate analyte . is determined following its separation from its matrix. Suspended solid: determination of solid that can be separated from the sample (filtration or extraction) . Example: barium sulfate, hydrous oxides
1.2 This method is suitable for all concentration ranges of sulfate (SO 4-2); however, in order to obtain reliable readings, use a sample aliquot containing not more than 40 mg/L of SO 4-2. 1.3 The minimum detectable limit is approximately 1 mg/L of SO 4-2. 2.0 SUMMARY OF METHOD 2.1 Sulfate ion is converted to a barium sulfate suspension underSulfate (Gravimetric) Current Revision. Issued 1974; Editorial Revision 1978: Media. . Methods for the Chemical Analysis of Water and Wastes (MCAWW) (EPA/600/4-79/020) . Occlusion of alkali sulfate with barium sulfate causes the substitution of an element of lower atomic weight than barium in the precipitate. Hydrogen sulfate of alkali .The chemical analysis of barium sulphate is covered by very similar test methods in both the US and EU pharmacopoeias. They involve qualitative identification tests for barium and sulphate, pH, heavy metals, limit tests for sulphide, acid-soluble substances and soluble barium salts plus a quantitative gravimetric assay for barium as the .
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
The purpose of the experiment is to quantitatively determine the amount of sulphate, in barium sulphate, by the gravimetric method. (C) Theory. . Test for complete precipitation by adding a few drops of barium chloride to clear the supernatant liquid. 2) Washing and Filtration of Barium Sulphate Precipitation .2. Suppose that a small portion of the sulfate precipitated as sodium sulfate rather than as barium sulfate. How would this affect the result of the analysis? 3. From the following list, identify the interfering species for the sulfate determination method used in this experiment: Mg2+, Pb2+, Na+, NO3-, Cl-, PO43-.This document specifies a barium sulfate gravimetric method for the determination of the sulfur content of iron ores. This method is applicable to a concentration range of a mass fraction of sulfur from 0,01 % to 3,9 % in natural iron ores, iron ore concentrates and agglomerates including sinter products.The Analysis of Barium Sulfate By EVELYN M. COCHRAN, ERVIN B. INSKIP, PERRY KING, and HOWARD W. ZIEGLER A method suitable for compendia1 assay of barium sulfate USP has been developed. Barium is separated as the carbonate and precipitated as the chromate. Calcium, strontium, and silica do not interfere. The calcium and strontium content may be
Assay— Accurately weigh not less than 0.58 g and not more than 0.62 g of Barium Sulfate in a tared platinum crucible, add 10 g of anhydrous sodium carbonate, and mix by rotating the crucible. Fuse over a blast burner until a clear melt is obtained, and heat for an additional 30 minutes.This video (Gravimetric titration part 2 | Assay of barium sulphate (BaSO4)) contains basic principle, reaction, procedure as well as calculations involved i.
Gravimetric analysis is a quantitative method in chemistry that involves determining the amount, or concentration, of a substance present in a sample based on the measurement of its mass. This .
gravimetric sulfate determination procedure
gravimetric sulfate determination diagram
Digital Charpy Impact Tester (5J) exporters
WEBBruna Marquezine é o mais novo nome a participar da Towel Series, uma famosa série de fotos de Mario Testino que traz diversos famosos posando apenas com toalhas .
assay of barium sulphate by gravimetric method|barium sulfate concentration